These are the waxes. Methane consists of one carbon atom with four hydrogen atoms stuck to it.
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry
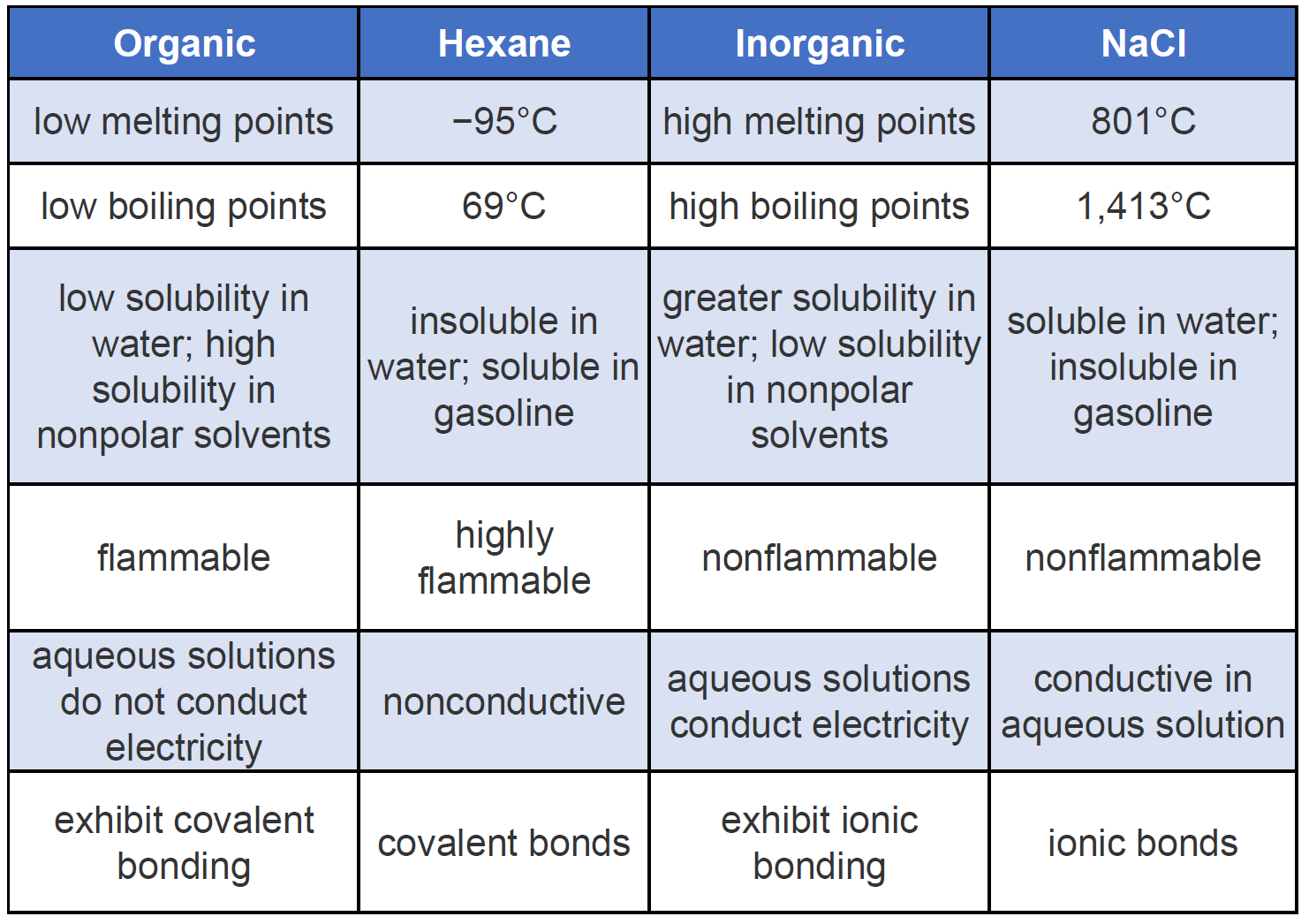
It is a highly flammable gas one of those that constitute natural gas and is capable of producing frostbite.

. As we considered organic structures in the earlier portions of this book alkanes were presented as examples because they are in many ways the simplest of organic molecules. Carbon makes double or triple bonds with other carbon atoms. What is Aliphatic Compound.
Methane Ethane Propane Butane and many more. The formation takes place with the help of single double or triple bonds. But they also have numerous uses in other fields.
We use hydrocarbons as solvents and fuels. This video describes in simple terms the property of resonance as well as the family of organic compounds based on benzene. Benzene is a cyclic hydrocarbon with the formula C 6 H 6.
Hydrocarbon gas liquids have many uses. Eventually a point is reached at which the materials are solids at room temperature. The unsaturated hydrocarbons contain multiple bonds.
Here are examples of the. They are not only major source of energy but also are materials used to make thousands of consumer products. Aromatic hydrocarbons are non-polar and relatively non-reactive due to these resonance structures and make excellent solvents.
In hydrocarbons carbon atoms are joined together to form the framework and the hydrogen atoms are attached to them in various configurations. It is a gas with a repulsive odor very flammable present in the atmosphere of the great gaseous planets and in ours it is the product of the decomposition of organic matter or a product of mining activities. Aromatic hydrocarbons also called aromatic compounds are compounds that contain benzene as a part of their structure.
Unsaturated hydrocarbons are compounds that consist only of the elements carbon C and hydrogen H and contain at least one carbon-carbon double or triple bond. Unsaturated hydrocarbons are extremely useful organic compounds in the manufacturing of plastics. These chemicals are essential for making plastics synthetic rubbers synthetic fibres.
Methane CH 4 ethane C 2 H 6 ethene C 2 H 4 and ethyne C 2 H 2 all are hydrocarbons as they are made up of only two elements. The boiling point of 1-octyne is higher than 1-pentyne. According to IUPAC rules the alcohol has the highest priority.
Organic chemical compounds that are made of carbon C and hydrogen H elements are known as hydrocarbons. Fossil fuels are hydrocarbons. A majority of aliphatic hydrocarbons are flammable.
Methane is a good example for aliphatic hydrocarbons with one atom of carbon and 4 atoms of hydrogen. The alkane CH 3 CH 2 388 CH 3 in which 390 carbon atoms are bonded in a continuous chain has been synthesized as an example of a so-called superlong alkane. The structure of aromatic hydrocarbons.
Hydrocarbons are the simplest type of carbon-based compounds. Several thousand carbon atoms are joined together in molecules of hydrocarbon polymers such as polyethylene polypropylene and polystyrene. We can find hydrocarbons in natural gas crude oil coal and plant life.
In this article you can learn about aliphatic hydrocarbons saturated and unsaturated hydrocarbons and their properties etc. In this formula N H is the number of hydrogen atoms while N C is the number of carbon atoms in the hydrocarbon molecule. The chemical compositions of HGL purity products HGL streams with a minimum of 90 of one type of HGL are similar but.
The main applications of hydrocarbons are given in transport as fuel and in industry. Polystyrene is used in making egg cartons disposable cups and other convenient products. The simplest hydrocarbon is methane.
Slightly larger hydrocarbons are known as kerosene or jet fuel diesel fuel and heating oil. Hydrocarbons vary greatly in size which influences properties such as melting and boiling points. These types of hydrocarbons are divided into three kinds.
Alkanes alkenes and alkynes. Hydrocarbons are compounds that contain only carbon and hydrogen. Still larger hydrocarbon molecules serve as lubricating oils and greases.
Hydrocarbons The alkanes and alkenes are examples of homologous series. At room temperature hydrocarbons may be gases liquids or solids. Hydrocarbon gas liquids HGLs are versatile products used in every end-use sectorresidential commercial industrial manufacturing and agriculture transportation and electric power.
Compare the boiling points of 1-pentyne and 1-octyne. Alkanes and cycloalkanes are examples of saturated hydrocarbon. These atoms are interlinked in a chain.
42 Names and Structures for Hydrocarbons. Hydrocarbons are the starting materials for the synthesis of organic chemicals of commercial importance. Up to 10 cash back Correct answer.
There are various types of hydrocarbons depending on how the molecules formed. This translates to counting the carbon chain from the left side giving six total carbons a hexane. LDPE which is a low-density variation of polyethylene is used in the manufacturing of grocery bags.
And yet hydrocarbons can be very small or very large can include straight chains of carbons or elaborate branching and can have. B Saturated hydrocarbons are those in which the carbon atoms are connected by single bonds and also called alkanes. Therefore the carbons should be counted to give this group the lowest carbon number.
The unsaturated hydrocarbons are of two types. Industrial chemicals such as alcohols include the usage. They are used in manufacturing fuels pesticides lacquers paints.
Gaseous hydrocarbons are methane and propane liquid hydrocarbons are hexane and benzene low melting solids or waxes hydrocarbons are paraffin wax and naphthalene and polymeric chains of hydrocarbons include polystyrene polypropylene and polyethylene. Compare the vapor pressure curves of 1-butene and 1-heptene. We can say that they are the basis of the current economy.
Benzene paraffin and methane for example are hydrocarbons. Unsaturated hydrocarbons are those in which the two carbon atoms are. Normal alkanes are the chain molecules depicted the first four examples in Table 201Once the number of carbon atoms reaches four butanes different permutations of an alkane molecule can exist that still honor Equation 201 but do not form.
Alkanes consist of a single covalent bond. In this way we find these components in the plastics insecticides and even cosmetics or soaps. Name each of the three types of unsaturated hydrocarbons summarize their structural differences and give an everyday source of each.
Ethylene isooctane and acetylene are examples of the same. Ethane C 2 H 6. A homologous series is a group of chemicals which have similar chemical properties and can be represented by a general formula.
Which are formed when different carbon atoms join to form an open-chain or a ringed structure.

General Introduction To Organic Compounds Properties Uses With Videos

Difference Between Crystalline And Amorphous Solids Http Www Curlyarrows Com Difference Crystalline Amo 11th Chemistry How To Memorize Things Physics Courses

Chemical Properties Of Alkanes What Are Examples Of Alkanes Chemical Property Chemistry Basics Chemical

0 Comments